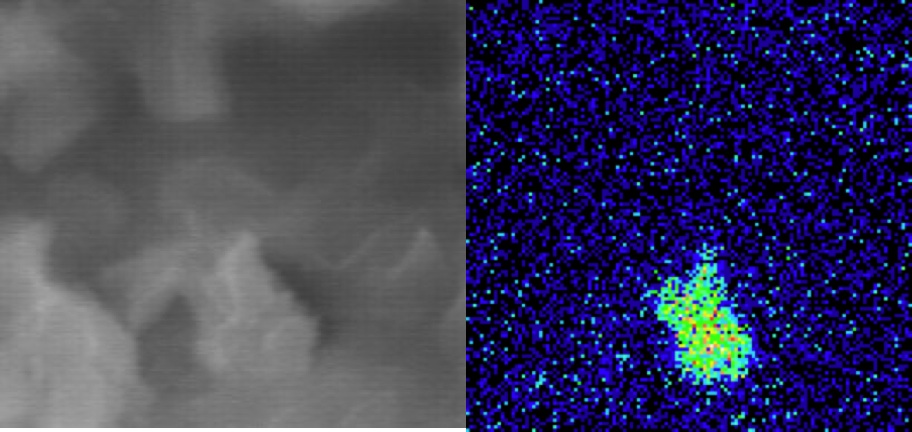
|
HOW PIMS USING APATITE IITM WORKS TO REMEDIATE SOIL & WATER
Phosphate-Induced Metal Stabilization (PIMSTM) using Apatite IITM stabilizes a wide range of metals, especially Pb, U, Cd, Zn, Cu and Al, in situ or ex situ, by chemically binding them into new phosphate minerals and other low-solubility phases that are stable over geologic time. The excellent stabilization efficiency comes from the extremely low solubility products (Ksp) of the resultant metal-apatites, e.g., for Pb-apatite (pyromorphite) Ksp ~ 10-80 (Nriagu, 1984; Ruby et al., 1994) to Ksp ~ 10-167 (Manecki et al., 2000). Combined with this thermodynamic stability, the rapid kinetics of the metal-phosphate precipitation and adsorption ensures immobilization of metals in the face of most transport mechanisms. Depending upon the metal, the concentration of the metal and the aqueous chemistry of the system, Apatite II works by four general, non-mutually-exclusive processes. This technology has been successful with contaminated range soils, groundwaters and wastewaters for Pb, U, Cd, Zn, Al and Cu, and has stabilized between 5% and 50% of its weight in metals depending upon the metal and the environmental conditions. Costs for range soil remediation are $20-$30/yd3 (see accompanying paper in this volume, Wright et al., 2004) and costs for water remediation are $40 per 1,000,000 gallons of water per mg/L of metal. Click Here to download a PDF of the full paper Pb Particle Sorbed onto a grain of Apatite II |